Regulatory Submissions. Simplified.

Where AI brings structure,
speed, and confidence.
Harmony is built to simplify and strengthen regulatory authoring for life sciences teams.
From creating CTDs to managing revisions and responding to regulator queries, Harmony supports
every stage of the regulatory lifecycle with clarity and control.

Where Submissions
Break Down
The hidden friction across regulatory workflows.

Manual CTD authoring
Heavy manual effort across drafting, formatting, and assembly.

Version control issues
Inconsistent updates across documents and teams.

Repeated regulator queries
Gaps and inconsistencies triggering avoidable rework.

Slow global submissions
Delays caused by region-specific requirements and coordination.

Siloed RA, QA, CMC teams
Disconnected workflows limiting visibility and collaboration.

Audit readiness stress
Last-minute checks to meet compliance expectations.
Built for the Future of
Regulatory Affairs
AI-powered capabilities designed to accelerate submissions
and strengthen compliance.

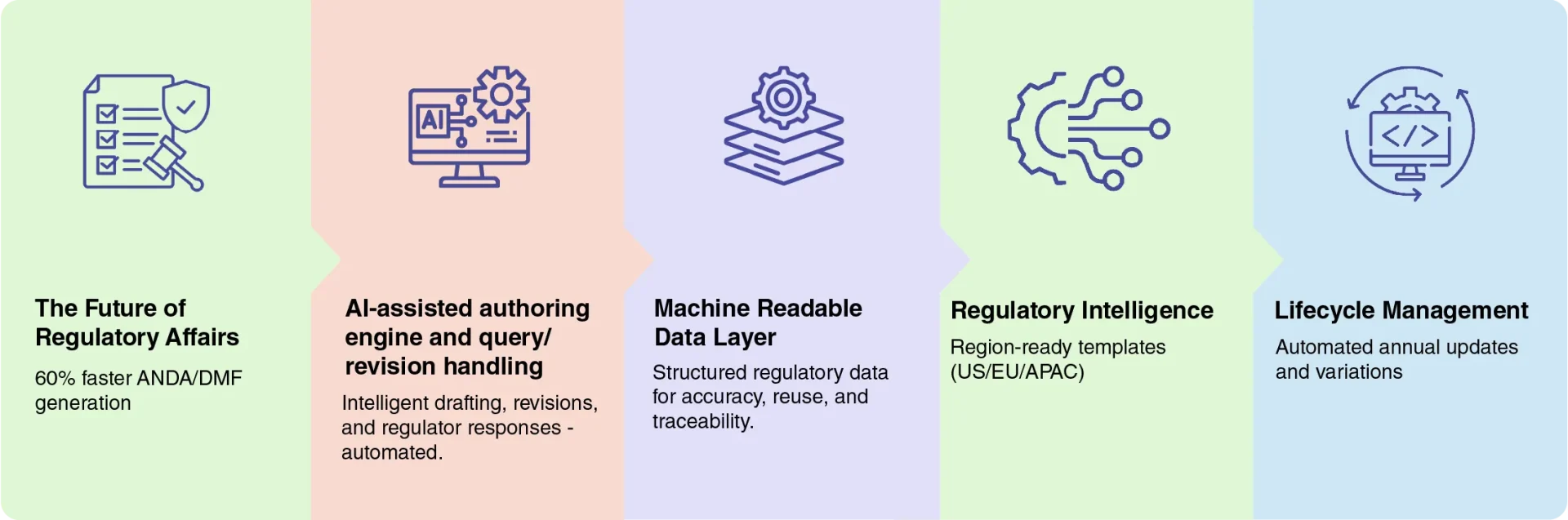
By automating regulatory authoring and lifecycle management, Harmony cuts submission time, reduces formatting effort, and accelerates QOS/QIS writing.

Built for regulated life sciences.
Designed for trust.
Built in close collaboration with regulatory experts, Harmony is designed to meet the real demands of life sciences teams.
By combining deep domain understanding with responsible AI, Harmony helps organizations move faster while maintaining trust, compliance, and control at every stage.

Life Sciences-Tuned LLMs
Models trained for regulatory language and domain accuracy.

21 CFR Part 11 Compliant
Built with audit trails, controls, and validation.

Global Region-Ready
Supports regional requirements across global markets.

Secure Private Deployment
Enterprise-grade security with isolated environments.
Strategic Benefits of AI-Powered Literature Search
40%
Faster Safety Reporting
70%
Reduction in Manual Workload
Precision and Accuracy
Eliminates manual errors by automating the extraction, validation, and classification of critical data. Smart Agents ensure higher quality.
Operational Efficiency
Handles large volumes of literature swiftly, drastically reducing processing time compared to traditional methods.
Regulatory Compliance
Ensures all reporting meets stringent compliance standards with validated and reliable data.
Scalable Solution
Adapts to growing data needs, enabling continuous monitoring and efficient data management over time.
Enhanced Focus
Frees up pharmacovigilance teams to concentrate on strategic initiatives, research, and in-depth analysis.
See the Impact Firsthand
Discover how our AI-Powered Literature Search Assistant can streamline your operations, enhance compliance, and deliver actionable insights with ease.
Transforming Literature Search for a Global Pharma Leader
Business Situation
A leading pharmaceutical company faced significant challenges in managing the manual review of a growing number of scientific publications.
This process was time-consuming and prone to human error, delaying critical safety evaluations and regulatory compliance.

The Solution
By implementing our AI-Powered Literature Search Assistant, the company automated key aspects of its literature search and validation process.
Smart agents, powered by large language models (LLMs), enabled rapid extraction and classification of article data, ensuring compliance with predefined MAH regions and patient type validations.
Outcome
- Reduced manual workload by over 70%, allowing teams to focus on high-value safety analysis.
- Accelerated safety reporting timelines by 40%, meeting stringent regulatory deadlines.
- Enhanced data accuracy and minimized human error, resulting in higher-quality safety assessments.
- Established a scalable framework for continuous monitoring and streamlined regulatory compliance.
Why choose us?
One-of-a-Kind RCA Solution for Life Sciences
Proven Expertise
Backed by a track record of successful implementations, our solution has consistently delivered measurable results for global pharma leaders.
Tailored for Life Sciences
Designed specifically to address the unique challenges of pharmacovigilance teams, ensuring relevance and impact.
Cutting-Edge AI
Leverage the latest advancements in AI and NLP to transform your literature search process with unparalleled precision.

End-to-End Automation
From data extraction to validation, our solution handles it all, reducing manual efforts and boosting efficiency.

Dedicated Support
Benefit from expert guidance and support at every step to ensure smooth deployment and ongoing success.
Not all AI platforms are built for regulated life sciences.
Most tools focus on publishing or workflows, adding AI as an afterthought. Harmony is designed AI-first, purpose-built for regulatory complexity.
| Capability | DeepForrest Harmony | Competitor 1 | Competitor 2 | Competitor 3 | Competitor 4 |
|---|---|---|---|---|---|
| AI Automation in Authoring | 55-75% AI-native authoring | 10-20% add-on LLMs | 5-15% rule-based | 30-45% templates | 15-25% assisted |
| eCTD Publishing | Planned integration | 70-85% full suite | 80-90% publishing-led | 20-30% partial | 60-75% integrated |
| Diff Checker Accuracy | 70-90% semantic, context-aware | 30-50% | 15-25% | 40-60% | 30-40% |
| Approach | AI-native RA platform | Platform + AI add-ons | Rules-driven | Template-based | Workflow-centric |
| Best For | Speed + intelligence | Publishing heavy teams | Compliance checks | Document assembly | Traditional RA ops |
Capability
DeepForrest Harmony
Competitor 1
Competitor 2
Competitor 3
Competitor 4
AI
Automation in Authoring
55–75% AI-native authoring
10–20% add-on LLMs
5–15% rule-based
30–45% templates
15–25% assisted
eCTD Publishing
Planned integration
70–85% full suite
80–90% publishing-led
20–30% partial
60–75% integrated
Diff Checker Accuracy
70–90% semantic, context-aware
30–50%
15–25%
40–60%
30–40%
Approach
AI-native RA platform
Platform + AI add-ons
Rules-driven
Template-based
Workflow-centric
Best For
Speed + intelligence
Publishing heavy teams
Compliance checks
Document assembly
Traditional RA ops
Flexible Pricing for
Regulatory Teams
Enterprise-ready pricing designed for regulatory scale, speed, and compliance
Our Success Stories That Speak for Themselves


Transforming Patient Feedback into Actionable Insights

Enhancing Patient Experiences Through NLP-Powered Chatbots